Respreeza® for Emphysema with A1-PI deficiency now Approved in European Union
Written by |
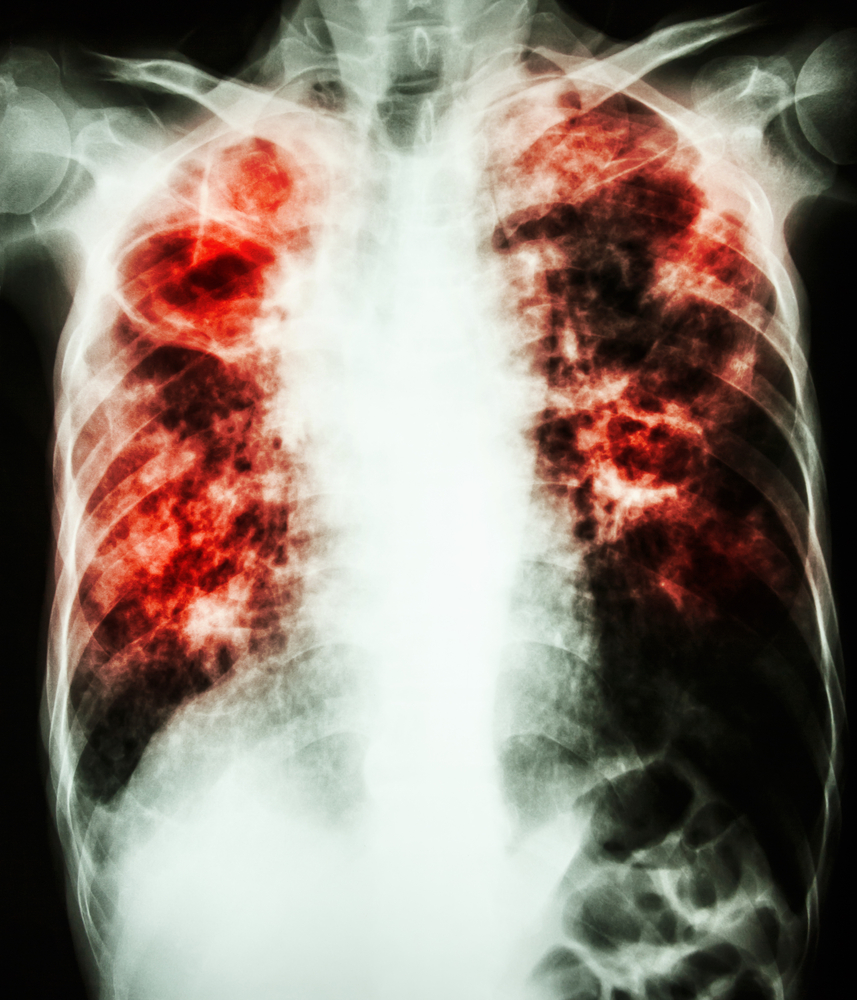
CSL Behring, a global leader in the plasma protein therapeutics industry, has just announced its breakthrough maintenance treatment for patients with emphysema with documented severe A1-PI deficiency (AATD). Respreeza® has received marketing authorization in all states in the European Union (EU). Respreeza is a highly purified Alpha-1 protein derived from human plasma and is the only Alpha-1 proteinase inhibitor that has been proven in a prospective double blind, placebo controlled trial (the RAPID study) to significantly reduce the loss of pulmonary tissue and slow the progression of emphysema.
Alpha-1 Antitrypsin Deficiency is an inherited condition that can result in severe lung disease in adults and liver disease at any age, as well as other less known manifestations such as paniculitis, a skin disease. AATD is the most commonly known genetic risk factor for emphysema and is commonly referred to as genetic COPD. Low levels or absence of the protective protein alpha-1 antitrypsin, which is produced by the liver, characterize AATD.
“AATD is a potentially debilitating disease and many affected individuals suffer from serious lung disease,” said Professor Helmut Teschler, MD, Director of the West German Lung Centre at the University of Essen. “With the approval of Respreeza®, healthcare professionals can now provide patients with severe AATD in Europe with a next generation Alpha1-proteinase inhibitor (A1-PI) that provided additional evidence that this augmentation therapy can slow the accelerated loss of lung tissue.”
Frank Willersinn, M.D., Alpha-1 Global Steering Committee Chair and patient representative in Europe said, “We are so glad that Respreeza® has been approved by the European Medicines Agency. We commend CSL Behring for their long-lasting commitment to the Alpha-1 community, now bringing their established Alpha 1-antitrypsin product to Europe, allowing it to be a cornerstone for treatment of AATD patients in the near future.”
A1-PI is not new to the US respiratory therapy market, however, as CSL Behring has been marketing the drug as Zemaira® for the past 12 years, indicated as a maintenance therapy for adult patients with A1-PI deficiency and clinical evidence of emphysema. In the US, Zemaira® is contraindicated in patients with a history of severe systemic reaction to the product or to any A1-PI protein. Because of the risk of severe hypersensitivity, Zemaira® is also contraindicated in immunoglobulin A (IgA)-deficient patients with antibodies against IgA.




